Atmosphere Layers
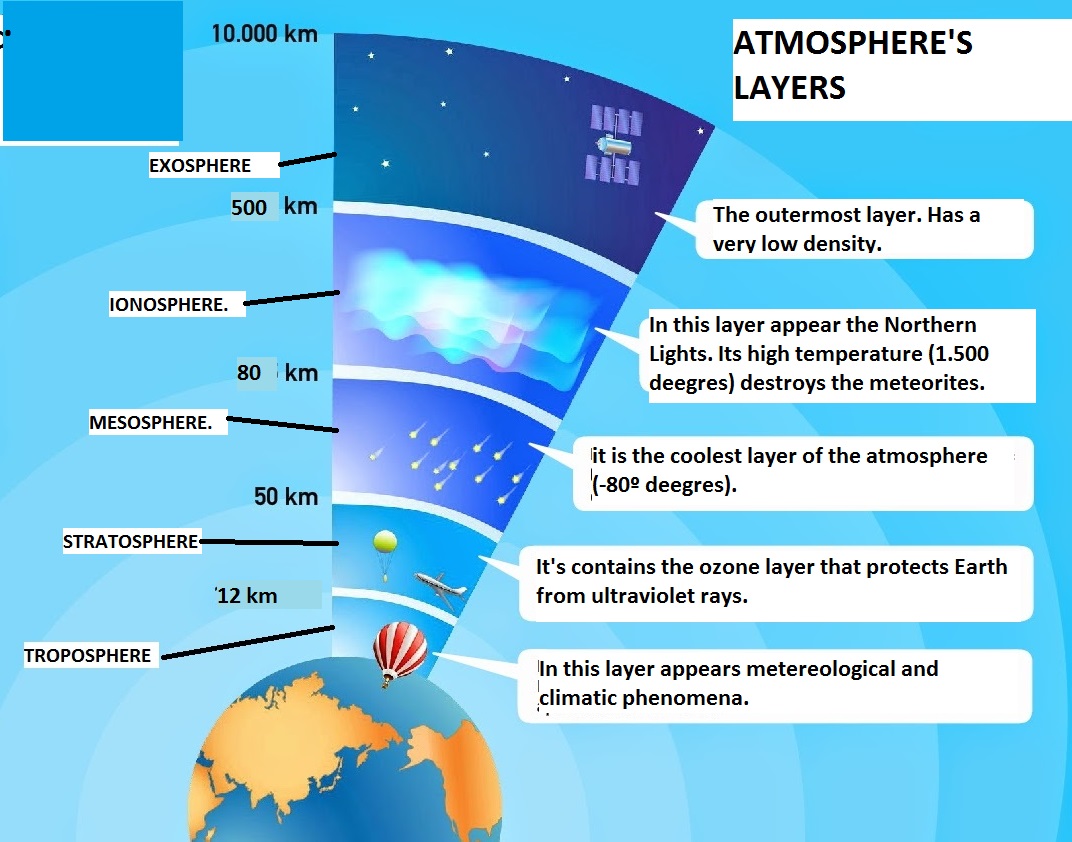
The Earth’s atmosphere has five distinct layers generally measured by temperature gradients, from the troposphere near the Earth's surface at an average 15 degrees Celsius to the temperature of the exosphere layer at 1,500 degrees Celsius. These layers create a habitable environment to sustain life.
The atmosphere is the layer of gases around the Earth. It is held in place by Earth's gravity. It is made up mainly of nitrogen (78.1%). It also has plentiful oxygen (20.9%) and small amounts of argon (0.9%), carbon dioxide (~ 0.035%), water vapor, and other gases. The atmosphere protects life on Earth by absorbing (taking) ultraviolet rays from the sun. It makes our days cooler and our nights warmer.
Solid particulates, including ash, dust, volcanic ash, etc. are small parts of atmosphere. They are important in making clouds and fog.
The atmosphere does not end at a specific place. The higher above the Earth, the thinner the atmosphere. There is no clear border between the atmosphere and outer space, though the Kármán line is sometimes treated as a border. 75% of the atmosphere is within 11 kilometres (6.8 miles) of the Earth's surface.
The 31st annual event, produced by Chicago-based Marketing and Media Solutions company Intersport, returns to Minneapolis for the first time since 2001.Twenty-four of the nation’s elite men’s and women’s college basketball players will convene in Minneapolis to participate in the event. :format(jpeg)/cdn.vox-cdn.com/uploads/chorus_image/image/53991259/519180590.0.jpg) The winners of the Men’s and Women’s 3-Point Championships will then compete in the Reese’s Puffs Battle of the Champions.“Every year, the State Farm College Slam Dunk & 3-Point Championships celebrates college basketball’s top seniors in an atmosphere that rivals any All-Star showcase,” said Intersport executive vice president Drew Russell. “It’s truly a family-friendly environment, where fans can experience the excitement of college basketball’s championship weekend with some of the most electric men and women in today’s game. The night features the Great Clips Slam Dunk Championship, the Men’s and Women’s 3-Point Shooting Championships and the Applebee’s Team Shootout. It promises to be another great event at Target Center.”Over the last 30 years, several of the top players in college basketball have competed in the College Slam Dunk & 3-Point Championships including: Gary Payton, Steve Nash, Jason Terry, Kyle Korver, Bobby Hurley, Michael Finley, Allan Houston, Wesley Matthews, Yogi Ferrell, Cappie Pondexter, Katie Gearlds, Kristi Toliver, Tiffany Hayes and Ariel Atkins.A complete roster of participants in the 2019 State Farm College Slam Dunk and 3-Point Championships will be released at a later date.
The winners of the Men’s and Women’s 3-Point Championships will then compete in the Reese’s Puffs Battle of the Champions.“Every year, the State Farm College Slam Dunk & 3-Point Championships celebrates college basketball’s top seniors in an atmosphere that rivals any All-Star showcase,” said Intersport executive vice president Drew Russell. “It’s truly a family-friendly environment, where fans can experience the excitement of college basketball’s championship weekend with some of the most electric men and women in today’s game. The night features the Great Clips Slam Dunk Championship, the Men’s and Women’s 3-Point Shooting Championships and the Applebee’s Team Shootout. It promises to be another great event at Target Center.”Over the last 30 years, several of the top players in college basketball have competed in the College Slam Dunk & 3-Point Championships including: Gary Payton, Steve Nash, Jason Terry, Kyle Korver, Bobby Hurley, Michael Finley, Allan Houston, Wesley Matthews, Yogi Ferrell, Cappie Pondexter, Katie Gearlds, Kristi Toliver, Tiffany Hayes and Ariel Atkins.A complete roster of participants in the 2019 State Farm College Slam Dunk and 3-Point Championships will be released at a later date.
History of Earth's atmosphere[changechange source]
Originally, the Earth's atmosphere had almost no free oxygen. The first atmosphere consisted of gases in the solar nebula, primarily hydrogen. There might be simple hydrides such as those now found in the gas giants (Jupiter and Saturn): water vapor, methane and ammonia.[1] The atmosphere gradually changed to mostly carbon dioxide and nitrogen. The lighter gases, like hydrogen and helium, cannot be held by the Earth's gravity, and would escape. For a long time (say 2 billion years or more), the atmosphere was dominated by carbon dioxide.
In the Great Oxygenation Event the atmosphere changed to the kind we have now, with oxygen replacing the carbon dioxide. Our atmosphere is still mostly nitrogen, but most living organisms interact more with oxygen than with nitrogen. Oxygenation began with cyanobacteria making free oxygen by photosynthesis. Most organisms today need oxygen for their respiration: only a few anaerobic organisms can grow without oxygen.[2][3]
Temperature and the atmospheric layers[changechange source]
Some parts of the atmosphere are hot or cold, depending on height. If something climbed straight up, it would get colder, but then it would get hotter as the object climbed higher. These changes of temperature are divided into layers. These are like layers of an onion. The difference between the layers is the way the temperature changes.
These are the layers of the atmosphere, starting from the ground:
- Troposphere - Starts at the ground. Ends somewhere between 0 to 18 kilometres (0 to 11 miles). The higher, the colder. Weather in this layer affects our daily life.
- Stratosphere - Starts at 18 kilometres (11 miles). Ends at 50 kilometres (31 miles). The higher, the hotter. The heat comes from the Ozone layer at the top of the stratosphere. There is little water vapor and other substances in this layer. Airplanes fly in this layer because it is usually stable and air resistance is small.
- Mesosphere - Starts at 50 kilometres (31 miles). Ends at 80 or 85 kilometres (50 or 53 miles). The higher, the colder. Winds in this layer are strong, so the temperature is not stable.
- Thermosphere - Starts at 80 or 85 kilometres (50 or 53 miles). Ends at 640 kilometres (400 miles) or higher. The higher, the hotter. This layer is very important in radiocommunication because it helps to reflect some radio waves.
- Exosphere - Above the thermosphere. This is the top layer, and merges into interplanetary space.
Where one layer changes to the next have been named '-pauses.' So the tropopause is where the troposphere ends (7 to 14 kilometres (4.3 to 8.7 miles) high). The stratopause is at the end of the stratosphere. The mesopause is at the end of the mesosphere. These are called boundaries.
The average temperature of the atmosphere at the surface of Earth is 14 °C (57 °F).
Pressure[changechange source]
The atmosphere has pressure. This is because even though air is a gas, it has weight. The average atmospheric pressure at sea level is about 101.4 kilopascals (14.71 psi).
Density and mass[changechange source]
The density of air at sea level is about 1.2 kilograms per cubic meter. This density becomes less at higher altitudes at the same rate that pressure becomes less. The total mass of the atmosphere is about 5.1 × 1018 kg, which is only a very small part of the Earth's total mass.
Related pages[changechange source]
References[changechange source]
- ↑Zahnle, K.; Schaefer, L.; Fegley, B. (2010). 'Earth's Earliest Atmospheres'. Cold Spring Harbor Perspectives in Biology. 2 (10): a004895. doi:10.1101/cshperspect.a004895. PMC2944365. PMID20573713.
- ↑Heinrich D. Holland: The oxygenation of the atmosphere and oceans. In: Phil. Trans. R. Soc. B, vol. 361, 2006, p. 903–915 http://rstb.royalsocietypublishing.org/content/361/1470/903.full.pdf
- ↑Knoll, Andrew H. 2004. Life on a young planet: the first three billion years of evolution on Earth. Princeton, N.J. ISBN0-691-12029-3